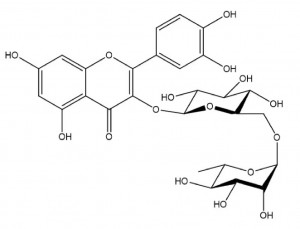
Rutin chemical formula is (C27H30O16•3H2O), a vitamin, has the effect of reducing capillary permeability and brittleness, maintain and restore the normal elasticity of capillaries. For prevention and treatment of hypertensive cerebral haemorrhage; Diabetic retinal hemorrhage and hemorrhagic purpura are also used as food antioxidants and pigments.
It is divided into the following four criteria:
1. Rutin NF11: yellow-green powder, or very fine acicular crystal; Odorless, tasteless; Color darkens in the air; Heated to 185-192 ℃, it becomes a brown gelatinous body and decomposes at about 215℃. Slightly soluble in boiling ethanol, slightly soluble in boiling water, very slightly soluble in cold water, easily soluble in isopropyl alcohol and methanol, insoluble in trichloromethane, ether and benzene; Soluble in alkali hydroxide solution. The identification method is A: hydrochloric acid reflux hydrolysis to quercetin, whose melting point is 312℃B: red cuprous oxide precipitation. C: Adding sodium hydroxide solution is orange yellow D: ethanol solution and ferric chloride solution is green brown E: ethanol solution with hydrochloric acid and magnesium gradually red content: ≥95.0%(UV)(by dry products)
Dry weight loss :5.5% ~ 9.0%
Burning residue ≤0.5%
Chlorophyll ≤0.004%
Red pigment ≤0.004%
Related substance quercetin ≤5.0%(UV)
Total number of aerobic bacteria ≤103cfu/g
Total number of mold and yeast ≤102cfu/g
Escherichia coli shall not be detected /g
Storage conditions Store in an airtight container away from light.
2. Rutoside Trihydrate E.P. 9.0: Yellow or yellow-green powder. Almost insoluble in water, soluble in methanol, slightly soluble in ethanol (96%), almost insoluble in methylene chloride. Soluble in hydroxide solution. The identification method is as follows :A: Maximum absorption at 257nm and 358nm, and maximum absorption coefficient at 358nm is 305 ~ 330. B: The infrared absorption pattern should be consistent with that of the reference product C: Spots of the same color and size will appear at the corresponding position of the chromatogram of the reference product D: ethanol solution with hydrochloric acid and zinc will show red
Content 95.0% ~ 101.0% (by dry product)(titration)
Moisture 7.5% ~ 9.5%(Cartesian)
Burning residue ≤0.1%
The maximum light absorption value of optical impurities at 450nm to 800nm shall not be greater than 0.10
Insoluble matter in methanol ≤3.0%
Related substance isoquercetin ≤2.0%, kaempferol-3-rutin ≤2.0%, quercetin ≤2.0%, total impurity ≤4.0%(HPLC)
Total number of aerobic bacteria ≤104cfu/g
Total number of mold and yeast ≤102cfu/g
Bile gram-negative bacteria ≤102cfu/g
Escherichia coli shall not be detected /g
Salmonella may not be detected /25g
Storage conditions keep away from light
3. Rutin USP43: The identification method is A: maximum absorption at 257nm and 358nm, and maximum absorption coefficient at 358nm is 305 ~ 33. B: The infrared absorption spectra should be consistent with the chromatogram of the reference product. C: The retention time of the chromatogram peak should be consistent with that of the reference product
Content 95.0% ~ 101.0% (by dry product)(titration)
Moisture 7.5% ~ 9.5%(Cartesian)
Burning residue ≤0.1%
The maximum light absorption value of optical impurities at 450nm to 800nm shall not be greater than 0.10
Insoluble matter in methanol ≤3.0%
Related substances isoquercetin ≤2.0%, kaempferol-3-rutin ≤2.0%, quercetin ≤2.0%, other mono-miscellaneous ≤1.0%, total impurity ≤4.0%(HPLC)
Total number of aerobic bacteria ≤104cfu/g
Total number of mold and yeast ≤103cfu/g
Escherichia coli shall not be detected /10g
Salmonella shall not be detected /10g
Storage condition sealed and kept away from light.
4. Ministry standard WS1-49(B)-89 of Rutinum: yellow or yellow-green powder, or very fine acicular crystal; Odorless, tasteless; Color darkens in the air; Heated to 185 ~ 192℃ to become a brown gel. Slightly soluble in boiling ethanol, slightly soluble in boiling water, very slightly soluble in cold water, insoluble in trichloromethane and ether; Soluble in alkali hydroxide solution. The identification method is: A: red cuprous oxide precipitate. B: The infrared absorption pattern should be consistent with that of the control substance. C: Maximum absorption is found at the wavelengths of 259±1nm and 362.5±1nm.
Content ≥93.0%(UV)(by dry product)
Dry weight loss 5.5% ~ 9.0%
Burning residue ≤0.3%
Insoluble matter in methanol ≤2.5%(insoluble matter in ethanol)
Related substance quercetin ≤4.0%(Thin layer)
Total number of aerobic bacteria ≤103cfu/g
Total number of mold and yeast ≤102cfu/g
Escherichia coli shall not be detected /g
Salmonella shall not be detected /g
Storage condition sealed and kept away from light.

Pharmacological effect:
Antifree radical action
Oxygen molecules are reduced in the form of single electrons in cell metabolism, and the O ions generated by the reduction of oxygen molecules in the form of single electrons will then produce H2O2 and highly toxic ·OH free radicals in the body, thus affecting the skin’s smoothness and even accelerating the skin aging. Moreover, adding rutin to the product can obviously remove the reactive oxygen species produced by cells. Rutin is a flavonoid compound, which is a strong antioxidant for scavenging free radicals. It can stop the chain reaction of free radicals, inhibit the peroxidation of polyunsaturated fatty acids on biofilms, remove lipid peroxidation products, protect the integrity of biofilms and subcellular structures, and play an important role in the body. [2]
Anti-lipid peroxidation
Lipid peroxidation refers to a series of oxidation processes caused by reactive oxygen species attacking polyunsaturated fatty acids in biofilms. Zhu Jianlin et al. determined and analyzed SOD activity in rats, content of free-radical lipid peroxidation product MDA, and content of Lipofuscin in the large liver, and found that rutin had an inhibitory effect on lipid peroxidation in castrated rats, and could inhibit the decline of antioxidant capacity of the antioxidant system in rats after castrated. Rutin can resist the decline of antioxidant capacity caused by the decline of endogenous estrogen and has antioxidant effect. High density lipoprotein (HDL) has anti-atherosclerotic effects. However, HDL, like low density lipoprotein (LDL) and very low density lipoprotein (VLDL), can also be oxidized and modified in vitro. Once HDL is oxidized and modified into Ox-HDL, it has the effect of atherosclerosis. Meng Fang et al. investigated the effect of rutin on HDL oxidative modification by Cu2+ mediated oxidative modification in vitro. Conclusion Rutin can significantly inhibit the oxidative modification of HDL. [2]
Antagonistic effect of platelet activating factor
The pathogenesis of many cardiovascular and cerebrovascular diseases, such as thrombosis, atherosclerosis, inflammatory reaction and ischemia-reperfusion free radical injury, are closely related to the mediation of plateletactivatingfactor (PAF). Therefore, antagonizing the effect of PAF is an important way to alleviate ischemic cardiovascular and cerebrovascular diseases. Experiments showed that rutin could antagonize the specific binding of PAF to rabbit platelet membrane receptors concentration-dependent, inhibit the PAf-mediated platelet adhesion in rabbits and the increase of free Ca2+ concentration in PMNs, suggesting that the mechanism of rutin’s anti-PAF action is to inhibit the activation of PAF receptor, and then block the reaction induced by PAF, so as to play a role in cardiovascular protection. The results showed that rutin was a PAF receptor antagonist. [2]
Anti-acute pancreatitis
Rutin can effectively prevent hypocalcemia and reduce Ca2+ concentration in pancreatic tissue. It was found that rutin can increase the content of phospholipase A2 (PLA2) in pancreatic tissue of rats, suggesting that rutin may inhibit the release and activation of PLA2 in pancreatic tissue. Rutin can effectively prevent the occurrence of hypocalcemia in AP rats, possibly by preventing Ca2+ influx and reducing Ca2+ overload in pancreatic tissue cells, thereby reducing the pathophysiological damage to AP. [2]
Reference:https://mp.weixin.qq.com
https://xueshu.baidu.com/usercenter/paper
For rutin, please contact us. We are waiting for you here at any time.
Post time: Dec-27-2022